Sequence analysis of 5’-untranslated regions of plant mRNAs
Alexey V. Kochetov*, Max V. Pilugin, Fedor V. Kolpakov, Vladimir N. Babenko, Nikolay A. Kolchanov, Vladimir K. Shumny
a
Institute of Cytology and Genetics SD RAS, Pr. Lavrentieva, Novosibirsk-90, RussiaKey words: plant mRNA, leader sequences, statistical analysis, translational properties
Introduction
The 5’-untranslated regions (i.e., the region upstream of the main initiation site) of the mature eukaryotic mRNAs are implied to play a crucial role in initiation and efficiency of translation (Kozak, 1992). It was previously shown that several features of the leader sequence can influence the mRNA translational efficiency: (a) the nucleotide sequence or context surrounding the AUG codon (Kozak, 1987a, 1987b, 1989b;); (b) leader length (Kozak, 1991b, 1991c); (c) secondary structure in the leader (Pelletier and Sonenberg, 1986; Kozak, 1986, 1989c); (d) the presence of AUGs upstream of the main initiation site (Kozak, 1989a; Futterer and Hohn, 1992). According to the scanning model (Kozak, 1989a), 40S ribosomal subunit enters at the 5’end of the mRNA and migrates linearly until it reaches the first AUG codon, whereupon a 60S subunit joins and translation begins. The efficiency of AUG codon recognition is modulated by the sequence context of the codon. For mammalian mRNAs (Kozak, 1987a, 1987b), the most crucial positions are a purine at position -3 and a guanine at position +4 (where the A in AUG is numbered as +1), other positions seem to be less important.
In most plant mRNAs surveyed (92% of 79 higher plant gene mRNAs), the 5’ proximal AUG is used as the initiation codon (Joshi, 1987). The consensus sequence for the context was UAAACAAUGGCU (Joshi, 1987) or AACAAUGGC (Lutcke et al., 1987). The influence of the sequence context on initiation in the plant cells has been verified by mutagenesis studies (Taylor et al., 1987; Lutcke et al., 1987; Kozak, 1989b; Kirsi and William, 1990; Guerineau et al., 1992; Dinesh-Kumar and Miller, 1993). Some workers concluded that the context of the initiation codon in plants is of minor importance (Lutcke et al., 1987; Kirsi and William, 1990), others have shown that it discriminates the level of initiation, similar to that in mammalian systems (Taylor et al., 1987; Kozak, 1989b; Guerineau et al., 1992; Dinesh-Kumar and Miller, 1993). Exact role of positions of the AUG context during translation under different conditions is still unclear (Kato et al., 1991; Futterer and Hohn, 1996).
Secondary structures and AUGs within 5’UTR hinder the scanning and, as a consequence, decrease the translational efficiency of the mRNA. mRNAs with excessive secondary structure in their 5’UTR are discriminated against by the translational machinery and inefficiently translated in both plant and animal cells (Pelletier and Sonenberg, 1985; Kozak, 1986, 1989c; Futterer and al., 1993; Basso et al.,1994). Similarly, the introduction of alternative initiation sites reduces downstream translation (Kozak, 1989a; Futterer and Hohn, 1992; 1996). In some cases, translation of mRNAs with long and structurized 5’UTRs may be regulated specifically (Fu et al., 1991; Koromilas et al., 1992). Upstream AUG codons and mini-ORFs may also be used for regulation of gene expression at the translation stage (Geballe and Morris, 1994; Futterer and Hohn, 1996).
The length of 5’UTR may contribute to the mRNA translational efficiency (Kozak, 1991b, 1991c). In general, longer leaders (e.g. 80 nt) result in higher translational rates in plant cells than shorter leaders (e.g. 10 nucleotides); however, mRNAs with leader sequence less than 10 nucleotides long can still be efficiently translated (Futterer and Hohn, 1996).
Only a small number of leader sequences of nuclear plant genes have been systematically compared (Joshi, 1987). Computer analysis of the sequences identified so far may be useful to reveal the 5’UTR features influencing the mRNA translational properties (e.g., Kozak, 1987; Lutcke et al., 1987; Joshi, 1987; Pesole et al., 1994; Joshi and Nguyen, 1995). In this paper, we report a detailed computer analysis of the 5’ leader regions of plant mRNA sequences.
Methods
Selection of the 5’UTR sequences for the present compilation
The EMBL (Release 49, 1996, pln.dat) database was used for this compilation. 5’UTR mRNA sequences were extracted using the program L_EXTR (Babenko et al., unpublished). This program allows the selective extraction of both full-sized (i.e., when genomic DNA clone was sequenced and the transcription start site was determined by experiments) and possibly incomplete (i.e., when the cDNA clone was sequenced) 5’UTRs. For the selection of full-sized 5’UTRs from the “DNA”-marked EMBL entries, key fields “CDS” and one of “mRNA”, “precursor_RNA”, “prim_transcript”, “5’UTR” were used; for the selection of possibly incomplete 5’UTRs from the “mRNA”-marked EMBL entries, the start points of the sequence and the CDS were used.
Thus, two databases were created: database D containing full-sized 5’UTRs and database R containing possibly incomplete 5’UTRs. To avoid the bias due to redundant sequence data in the statistical analysis, both 5’UTR and CDS databases were checked for redundancies. The similarity scores for all the sequence pairs compared were determined. If two sequences in database shared a similarity greater than 70%, the shorter sequence was removed from the collection, the longer one was retained for further analysis. As we found, almost all the sequence pairs with a similarity greater than 70% belong to the same gene family. Some 5’UTRs were checked manually using the published information (for example, when the EMBL entry contained contradicting data or the leader length was unusually long).
The description of the sequence data is shown in Table 1.
Table 1. Summary description of 5’UTR databases used in the study
Full-sized 5’UTRs (D) |
Incomplete 5’UTRs (R) |
|
Extracted sequences |
572 |
4266* |
Redundant sequences |
93 |
856 |
Non-redundant sequences |
479 |
3410 |
Sequences of: - angiosperms - dicots - monocots - others |
454 333 121 25 |
3223 2475 748 187 |
* length > 15 nt
A small database of 3’UTR mRNA of plant genes (D3’) was also created. Trailer sequences were extracted from the same EMBL entries that were taken for the full-sized 5’UTR database (D). The total number of the 3’UTR mRNAs analyzed was 315 (217 sequences of dicots and 82 sequences of monocots).
The difference between the distributions of the characteristics of the nucleotide sequences were analyzed for statistical significance by the Kolmogorov-Smirnov test.
Results and discussion
1. Length of 5’UTRs
Mean lengths of the dicot and monocot mRNA leader sequences are given in the Table 2. As was shown, 5’UTRs of monocot mRNAs are generally longer than that of dicot mRNAs.
Table 2
The length of 5’UTR mRNAs of dicot and monocot plants
Sample |
Means, nt |
Std.Dev. |
Min, nt |
Max, nt |
N |
D (dicots) |
98.1 |
99.6 |
11 |
1140 |
333 |
D (monocots) |
113.1 |
113.7 |
13 |
931 |
121 |
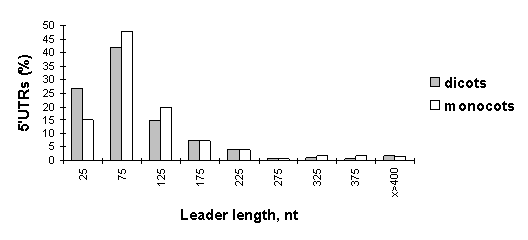
The distributions of the leader lengths of the dicot and monocot mRNAs are shown in the histogram (Fig. 1). 69% of the 5’UTRs of dicot mRNAs are shorter than 99 nt. In the case of monocots, the lengths of 67.7% of the 5’UTRs vary from 50 to 149 nt. The difference between the two distributions is significant (Ð<0.05).

Fig. 1. Histogram of size distribution of the 5’UTR sequences of dicot and monocot mRNAs (sample D).
2. Base composition
Nucleotide content in the 5’UTRs of dicot and monocot mRNAs is reported in Table 3. According to these results, 5’UTR sequences of dicot mRNAs are AU-rich sequences, whereas those of monocot mRNAs are C-rich.
Table 3. Nucleotide content in the untranslated sequences of plant mRNAs (samples R and D3’)
Dicot mRNAs |
Monocot mRNAs |
|||||||
5’UTRs |
3’UTRs |
5’UTRs |
3’UTRs |
|||||
Means |
Std.Dev. |
Means |
Std.Dev. |
Means |
Std.Dev. |
Means |
Std.Dev. |
|
A |
32.12 |
11.65 |
29.99 |
5.36 |
23.36 |
9.80 |
26.10 |
5.02 |
G |
15.64 |
8.40 |
17.03 |
3.32 |
23.46 |
8.66 |
22.24 |
4.51 |
C |
22.72 |
8.86 |
14.44 |
3.93 |
33.22 |
11.07 |
19.85 |
3.35 |
U |
29.52 |
10.53 |
38.52 |
5.17 |
19.95 |
8.43 |
31.80 |
4.05 |
AU |
61.64 |
9.26 |
68.51 |
4.29 |
43.32 |
11.38 |
57.90 |
5.68 |
AU/GC |
1.80 |
1.04 |
2.24 |
0.49 |
0.85 |
0.44 |
1.42 |
0.35 |
It is considered that GC richness of a 5’UTR sequence indicates the stability of the potential secondary structure, the formation of which may hinder the movement of 40S ribosomal subunit along mRNA during the scanning process (Kozak, 1991; 1992). We analyzed the 5’UTR samples for the ratios of the concentrations of the complementary nucleotides involved in the formation of the secondary structure (G/C and A/U). This parameter appeared of interest because the asymmetry of the A/U and G/C ratios would lead to a decrease in the stability of the mRNA secondary structure. The secondary structure would also be less stable, if G/C (A/U)>1 and G/C (A/U)<1. The ratios of the concentrations of the complementary nucleotides in the untranslated sequences of plant mRNAs are shown in Table 4.
Table 4. The ratios of the complementary nucleotides in the untranslated sequences of plant mRNAs (samples R and D3’)*
Number of 5’UTRs (sample R,%) |
Number of 3’UTRs(sample D3’,%) |
||||||||||
Dicots |
Monocots |
Dicots |
Monocots |
||||||||
x |
G/C |
A/U |
G/C |
A/U |
G/C |
A/U |
G/C |
A/U |
|||
x<0.2 |
10.30 |
0.77 |
2.54 |
1.34 |
0.00 |
0.00 |
0.00 |
0.00 |
|||
0.2<=x<0.4 |
17.09 |
5.09 |
15.24 |
4.81 |
0.00 |
1.84 |
0.00 |
0.00 |
|||
0.4<=x<0.6 |
17.86 |
12.36 |
20.85 |
10.83 |
4.61 |
13.82 |
1.22 |
10.98 |
|||
0.6<=x<0.8 |
14.38 |
16.97 |
20.19 |
11.90 |
10.60 |
37.79 |
12.20 |
39.02 |
|||
0.8<=x<1.0 |
9.33 |
12.97 |
13.50 |
11.63 |
11.98 |
29.03 |
18.29 |
28.05 |
|||
1.0<=x<1.2 |
9.94 |
11.07 |
9.76 |
11.76 |
23.96 |
12.44 |
25.61 |
14.63 |
|||
1.2<=x<1.4 |
4.00 |
8.52 |
5.75 |
9.22 |
15.21 |
4.15 |
25.61 |
6.098 |
|||
1.4<=x<1.6 |
3.23 |
5.78 |
4.14 |
7.89 |
13.36 |
0.00 |
10.98 |
1.22 |
|||
1.6<=x<1.8 |
2.75 |
4.77 |
1.87 |
4.68 |
8.29 |
0.46 |
3.66 |
0 |
|||
1.8<=x<2.0 |
1.45 |
2.59 |
0.94 |
4.81 |
2.76 |
0.00 |
2.44 |
0 |
|||
x>2.0 |
9.65 |
20.11 |
5.2 |
21.12 |
9.12 |
0.46 |
0 |
0 |
|||
*Designations: x, the G/C or A/U value;
As the data in the Table 4 show, the G and C concentrations are close (0.8<=G/C<1.2) only in 19.27% of the leaders in the sample of the dicot mRNAs, whereas they are 35.94% in the corresponding sample of the 3’UTRs. Similar pattern was observed for the A/U ratio: the A and U concentrations were close (0.8<=A/U<1.2) in 24.04% of the 5’UTRs of the dicot mRNAs and in 41.47% of the 3’UTRs. The same patterns were found for the G/C and A/U concentration ratios in the untranslated sequences of monocot mRNAs (Table 4). The differences in the distributions of the A/U and G/C ratios between the 5’UTRs and 3’UTRs of plant mRNAs are significant (P<0.001).
3. Context sequence of the AUG codon
Sequences flanking the AUG codon are believed to be important for the efficiency of the translation initiation (Kozak, 1989a; Futterer and Hohn, 1996; Gallie, 1993). When the context of the AUG codon is unfavorable, a part of the 40S ribosomal subunits does not recognize it as the translation start, thereby reducing the rate of polypeptide synthesis. The consensus sequence for the AUG context in plant mRNAs was previously defined as UAAACAAUGGCU (Joshi, 1987) or AACAAUGGC (Lutcke et al., 1987).
Table ??3 shows the frequencies of nucleotide occurrence in the region 12 nt upstream and 3 nt downstream around the functional AUG codons in dicot and monocot mRNAs. The differences of nucleotide frequencies in several context positions appeared to be considerable.
Table ??3. Nucleotide frequencies at positions flanking the AUG codon in dicot and monocot mRNAs. Pos, the positions of the nucleotides in the context relative to the AUG codon (A in AUG is assigned number 1).
Dicot mRNAs |
Monocot mRNAs |
||||||||
Pos |
A,% |
G,% |
C,% |
U,% |
A,% |
G,% |
C,% |
U,% |
|
-12 |
33,7 |
17,8 |
19,6 |
28,9 |
25,7 |
28,1 |
26,7 |
19,5 |
|
-11 |
33,7 |
18,7 |
19,9 |
27,8 |
26,7 |
28,5 |
27,9 |
16,8 |
|
-10 |
36,1 |
18,0 |
18,0 |
27,9 |
25,4 |
30,0 |
25,8 |
18,9 |
|
-9 |
32,9 |
18,9 |
18,1 |
30,1 |
21,9 |
31,3 |
27,5 |
19,3 |
|
-8 |
33,3 |
15,3 |
22,9 |
28,6 |
24,5 |
22,9 |
34,2 |
18,5 |
|
-7 |
36,0 |
17,3 |
18,4 |
28,3 |
25,0 |
29,8 |
26,7 |
18,5 |
|
-6 |
34,7 |
22,0 |
15,6 |
27,7 |
22,5 |
38,8 |
19,5 |
19,3 |
|
-5 |
30,7 |
16,1 |
26,9 |
26,4 |
19,0 |
18,2 |
41,7 |
21,1 |
|
-4 |
46,9 |
17,9 |
18,3 |
17,0 |
33,0 |
21,9 |
35,0 |
10.0 |
|
-3 |
56,7 |
23,6 |
8,1 |
11,6 |
36,6 |
44,3 |
10,2 |
8,9 |
|
-2 |
44,2 |
7,6 |
29,5 |
18,7 |
24,6 |
12,3 |
53,1 |
10.0 |
|
-1 |
45,7 |
18,2 |
23,1 |
13,0 |
23,0 |
30,1 |
42,8 |
4,14 |
|
+4 |
15,7 |
67,3 |
5,21 |
11,8 |
11,8 |
70,2 |
7,62 |
10,4 |
|
+5 |
22,6 |
15,2 |
51,4 |
11,0 |
19,7 |
14,6 |
59,1 |
6,68 |
|
+6 |
21,1 |
30,1 |
11,4 |
37,5 |
8,69 |
52,9 |
19,1 |
19,3 |
|
Means |
32.12 |
15.64 |
22.72 |
29.52 |
23.36 |
23.46 |
33.22 |
19.95 |
|
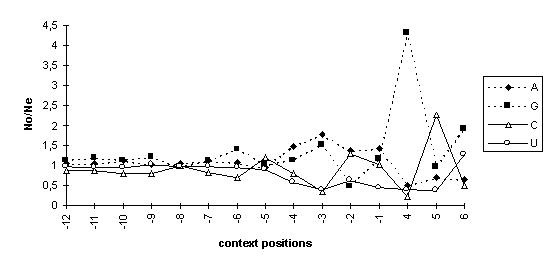
As the data in the Table show, consensus sequences of the AUG codon contexts in dicot and monocot mRNAs are different. The ratios of the observed nucleotide frequencies in positions of the AUG codon contexts (No) and the means in the corresponding samples of the 5’UTRs (Ne) demonstrated the nonrandom character of the context sequences (Fig. 2). The significant deviations of the observed nucleotide frequencies from the expected frequencies are found in the context positions -6, -4, -3, -2, -1, +4, +5, and +6 around the AUG codons in both dicot (Fig 2A) and monocot (Fig 2B) mRNAs.
A)
B)
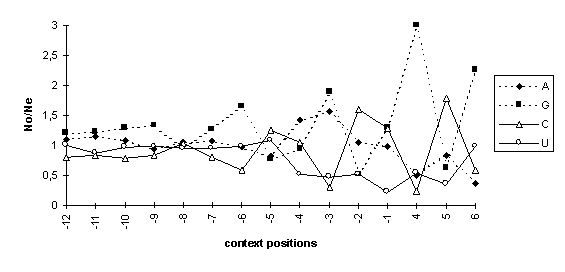
Fig. 2. The ratios of observed/expected nucleotide frequencies in the AUG codon context of dicot (A) and monocot (B) mRNAs. No/Ne, the ratio of the observed (o, observed) nucleotide frequency in a context position to its mean (e, expected) frequency in 5’UTRs of the corresponding sample.
The increases in guanine frequencies in positions -6 and -3 are similar to that in the consensus sequence of the AUG codon contexts of vertebrate mRNAs (Kozak, 1987). The increases in guanine frequency in positions -6, -3, and +4 are accompanied with the decreases in cytidine frequency; in positions -5, -2, and +5, the increase in cytidine and the decrease in guanine occur.
It had been previously reported that the frequencies of guanine and cytidine in position -3 before AUG in monocot mRNAs are very close (Cavener and Ray, 1991). Our results support this observation (the frequencies of G and A in position -3 are 44,3% and 33.6%, respectively). It may be possible that the effects of adenine and guanine in position -3 before AUG on the context “strength” are equal. High concentrations of pyrimidines in this position in both dicot and monocot mRNAs (19.7% and 19.1%, respectively) may indicate that the nucleotides in other positions (i.e., +4, +5) make comparable contribution to the AUG context “strength”, and the effect of nonoptimal pyrimidines in position -3 may be compensated by their positive influence.
Consensus sequences of the AUG codon contexts in dicot and monocot mRNAs are listed in Table ??4.
Table 4. Consensus sequences of the AUG codon contexts in dicot and monocot mRNAs (Pos., positions around AUG)
Positions |
-10 |
-9 |
-8 |
-7 |
-6 |
-5 |
-4 |
-3 |
-2 |
-1 |
+4 |
+5 |
+6 |
Dicot mRNAs |
|||||||||||||
Over-represented |
- |
- |
- |
- |
- |
- |
A |
A,g |
A,c |
A |
G |
C |
U,G |
Under-represented |
U |
U,C |
G,U |
U |
C,A,U |
A,U |
C,A |
||||||
Monocot mRNAs |
|||||||||||||
Over-represented |
- |
- |
- |
- |
G |
C |
- |
G,A |
C |
C,G |
G |
C |
G |
Under-represented |
C |
- |
U,C |
G,U |
U |
C,A,U |
U,G |
A,C |
|||||
According to the scanning model (Kozak, 1989a), the nucleotides in -3 and +4 positions make a major contribution to the translation from the AUG codon. We analyzed the frequencies of contexts with different combinations of nucleotides in these positions around AUG codons in the samples of dicot and monocot mRNAs. The results obtained are reported in the Table 5.
Table ??5. Frequencies of the mRNAs containing different combinations of nucleotides in positions -3 and +4 around the AUG codon: (1), A-3, G+4; (2) G-3 , G+4; (3) Py-3, G+4; (4) A-3, not G+4; (5) G-3 , not G+4; and (6) Py-3, Py+4
Dicot mRNAs |
Monocot mRNAs |
|||
AUG type (Nu-3;Nu+4) |
Sample D,% |
Sample R,% |
Sample D,% |
Sample R,% |
1 (A; G) |
42.6 |
35.9 |
31.4 |
19.8 |
2 (G; G) |
12.7 |
16.5 |
41.3 |
35.3 |
3 (Py; G) |
14.7 |
14.9 |
7.4 |
15.1 |
4 (A; not G) |
21.9 |
20.8 |
11.6 |
16.8 |
5 (G; not G) |
5.4 |
7.1 |
5.8 |
9.0 |
6 (Py; Py) |
2.7 |
4.8 |
2.5 |
4.0 |
It was found that the frequencies of the AUG codon containing guanine in positions -3 and +4 were different for dicot and monocot mRNAs. It is likely that G-3/G+4 combination provides at least equal contribution to the AUG translational activity as A-3/G+4 in monocots. A higher frequency of combination A-3/ not G+4 over the G-3/not G+4 may be explained by the dependence of G-3 activity on the guanine in position +4.
4. The presence of AUG codons in 5’UTRs
It was shown that upstream AUG codons decrease the mRNA translational efficiency in eukaryotic cells (Kozak, 1989a; Futterer and Hohn, 1992; ...). The frequencies of AUGs in the leader sequences of plant mRNAs are listed in the Table 6.
Table 6. The frequency of AUG codons in 5’UTRs of plant mRNAs
5’UTR features |
Dicots |
Monocots |
|||
Sample D |
Sample R |
Sample D |
Sample R |
||
Number of 5’UTRs |
333 |
2475 |
121 |
748 |
|
| Number of AUG-containing 5’UTRs | 65 (19.5%) |
465(18.8%) |
23 (19.0%) |
97 (13.0%) |
|
Number of 5’UTRs containing: 1 AUG (Nu-3; Nu+4): (A; G) (G; G) (Py; G) (A; not G) (G; not G) (Py; Py) 2 AUGs 3 AUGs 4 AUGs >4 AUGs |
29 (8.7%) 0 2 3 8 1 15 20 (6%) 5 (1.5%) 8 (1.7%) 3 (0.9%) |
241(9.7%) 21 13 39 41 18 109 98 (4%) 44 (1.8%) 21 (0.8%) 61 (2.5%) |
16 (13.2%) 0 0 3 2 0 11 2 (1.7%) 2 (1.7%) 0 (0%) 3 (2.5%) |
47 (6.3%) 3 6 5 7 7 19 17 (2.3%) 17 (2.3%) 4 (0.5%) 12 (1.6%) |
|
| Number of 5’UTRs (%) containing AUGs only
in nonoptimal context (Py-3, Py+4) |
20 (6%) |
134 (5.4%) |
12 (9.9%) |
24 (3.2%) |
|
The most frequent occurrence of the upstream AUGs flanked with pyrimidines was recorded in both dicot and monocot 5’UTR mRNAs. According to the scanning model, the upstream AUG in a favorable context can significantly decrease translation of the main ORF (Kozak, 1989a, 1991). It seems likely that AUG codons in the favorable contexts should be eliminated from the 5’UTRs of the functional mRNAs. The frequencies of the AUGs in different contexts (Nu-3; Nu+4) may be approximately ranged as: (A-3, G+4)= (G-3 , G+4) <=(G-3 , not G+4)<(A-3, not G+4)=(Py-3, G+4)<<(Py-3, Py+4) in 5’UTRs of dicot mRNAs and as (A-3, G+4)= (G-3, G+4) < (A-3, not G+4) = (G-3 , not G+4) = (Py-3, G+4)<<(Py-3, Py+4) in 5’UTRs of monocot mRNAs.
Conclusions
1. The lengths of the majority (67.7%) of the leaders of monocot mRNAs vary from the 50 to 149 nt; most part of the 5’UTRs of dicot mRNAs (69%) are shorter than 100 nt.
2. 5’UTR sequences of dicot mRNAs are AU-rich sequences, whereas those of monocot mRNAs are C-rich.
3. Leader sequences of both dicot and monocot mRNAs are much more asymmetrical in the content of complementary nucleotides compared with the 3’UTRs.
4. The frequencies of nucleotides in several positions around the start AUG codon of the dicot and monocot mRNAs are different. It is possible that functional activities of the nucleotides in certain positions of the AUG contexts differ both between mRNAs of plants/animals and monocot/dicot plants.
5. 5’UTRs of dicot mRNAs and (to a lesser extent) monocot mRNAs often contain AUG codons (13-19% of leaders). Only part of these upstream AUGs lie in unfavorable contexts (Table 6). It seems likely that upstream AUGs decrease the mRNA translation efficiency in the plant cells to a lesser extent compared with the vertebrate cells. It is unclear how the ribosomes discriminate between the functional and false translation starts in these mRNAs; may be, there are additional signals within 5’UTRs influencing the recognition of AUG codons (Kato et al., 1991; Kirsi and Williams, 1990).
References
Basso J., Dallair P., Charest P.J., Devantier Y., Laliberte J.-F. Evidence for an internal ribosome entry site within the 5'non-translated region of turnip yellow mosaic virus // J. Gen. Virol. 1994. V. 75. P. 3157-3165.
Bernardi G. The vertebrate genome: isochores and evolution // Mol. Biol. Evol. 1993. V. 10. P. 186-204.
Browning K.S., Lax S.R., Hamphreys J., Ravel J.M., Jobling S.A., Gehrke L. Evidence that the 5’-untranslated leader of mRNA affects the requirement for wheat germ initiation factors 4A, 4F, and 4G // J. Biol. Chem. 1988. V. 263. P. 9630- 9634.
Dinesh-Kumar S. P., Miller W. A. Control of start codon choice on a plant viral RNA encoding overlapping genes. // Plant Cell. 1993. V. 5. P. 679-692.
Fu L., Ye R., Browder L.W., Johnston R.N. Translational properties of messenger RNA with secondary structure in Xenopus // Science. 1991. V. 251. P. 807-810.
Futterer J., Hohn Th. Role of an upstream open reading frame in the translation of polycistronic mRNAs in plant cells // Nucleic Acids Res. 1992. V. 20. P. 3851-3857.
Futterer J., Kiss-Laszlo Z., Hohn T. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA // Cell. 1993. V. 73. P. 789-802.
Futterer J., Hohn Th. Translation in plants - rules and exceptions // Plant Mol. Biol. 1996. V. 32. P. 159-189.
Gallie D.R., Sleat D.E., Watts J.W.,Turner P.C., Wilson T.M.A. A comparison of eukaryotic viral leader sequences as enhancers of mRNA expression in vivo // Nucleic Acids Res. 1987. V. 15. P. 8693-8711.
Gallie D.R.,Walbot V. Identification of the motifs within the tobacco mosaic virus 5’-leader responsible for enhancing translation // Nucleic Acids Res. 1992. V. 20. P. 4631-4638.
Gallie D.R., Young T.E. The regulation of gene expression in transformed maize aleurone and endosperm protoplasts // Plant Physiol. 1994. V. 106. P. 929-939.
Geballe A.P., Morris D.R. Initiation codons within 5’-leaders of mRNAs as regulators of translation//Trends Biochem. Sci. 1994. V. 19. P. 159-164.
Guerineau F., Lucy A., Mullineaux P. Effect of two consensus sequences preceding the translation initiator codon on gene expression in plant protoplasts // Plant Mol. Biol. 1992. V. 18. P. 815-818.
Hess M. A., Duncan R. F. RNA/protein interactions in the 5'-untranslated leader of HSP70 mRNA in Drosophila lysates. Lack of evidence for specific protein binding // J. Biol. Chem. 1994. V. 269. P. 10913-10922.
Joshi C.P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes // Nucleic Acids Res. 1987. V. 15. P. 6643-6652.
Joshi C.P., Nguen H.T. 5' untranslated leader sequences of eukaryotic mRNAs encoding heat shock induced proteins // Nucleic Acids Res. 1995. V. 23. P. 541-549.
Kato T., Shirano Y., Kawazu T., Tada Y., Itoh E., Shibata D. A modified beta-glucuronidase gene: sensitive detection of plant promoter activities in suspension-cultured cells of tobacco and rice // Plant Mol. Biol. Rep. 1991. V.9. P. 333-339.
Kirsi L., William D. Changing the start codon context of the 30K gene TMV from "weak" to "strong" does not increase expression // Virology. 1990. V. 174. P. 169-176.
Koromilas A.E., Lazaris-Karatzas A., Sonenberg N. mRNAs containing extensive secondary structure in their 5’ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E // EMBO J. 1992. V. 11. P. 4153-4158.
Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes // Proc.Natl.Acad.Sci. USA. 1986. V. 83. P. 2850-2854.
Kozak M. An analysis of 5’- noncoding sequences from 699 vertebrate messenger RNAs // Nucleic Acids Res. 1987. V. 15. P. 8125-8148.
Kozak M. At least 6 nucleotides preceding the AUG initiator codon enhance translation in mammalian cells // J. Mol. Biol. 1987. V. 196. P. 947-950.
Kozak M: The scanning model for translation: an update // J. Cell Biol. 1989. V. 108 P. 229-241.
Kozak M. Context effects and inefficient initiation at non-AUG codons in eukaryotic cell-free translation systems // Mol. Cel. Biol. 1989. V. 9. P. 5073-5080.
Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eukaryotic mRNAs // Mol.Cell.Biol. 1989. V. 9. P. 5134-5142.
Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control// J. Cell Biol. 1991. V. 115. P. 887-903.
Kozak M. Effect of long 5’ leader sequence on initiation by eukaryotic ribosomes in vitro // Gene. Exp. 1991. V. 1. P. 117-125.
Kozak M: Structural features in eukaryotic mRNAs that modulate the initiation of translation // J. Biol. Chem. 1991. V. 266. P. 19867-19870.
Kozak M. Regulation of translation in eukaryotic systems // Annu. Rev. Cell. Biol. 1992.
Looman A.C., Kuivenhoven J.A. Influence of the three nucleotide upstream of theinitiation codon on the expression of the E.coli lacZ gene in Saccharomyces cerevisiae // Nucleic Acids Res. 1993. V. 21. P. 4268-4271.
Lutcke H.A., Chow K.C., Mickel F.S., Moss K.A., Kern H.F., Scheele G.A. Selection of AUG initiation codons differs in plants and animals // EMBO J. 1987. V. 6. P. 43-48.
Matassi G., Montero L.M., Salinas J., Bernardi G. The isochore organization and the compositional distribution of homologous coding sequences in the nuclear genome of plants // Nucleic Acids Res. 1989. V. 17. P. 5273-5290.
Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency // Cell. 1985. V. 40. P. 515-526.
Pesole G., Fiormarino G., Saccone C. Sequence analysis and compositional properties of untranslated regions of human mRNAs // Gene. 1994. V. 140. P. 219-225.
Pitto L., Gallie D.R., Walbot V. Role of the leader sequence during thermal repression of translation in maize, tobacco, and carrot protoplasts // Plant Physiol. 1992. V. 100. P. 1827-1833.
Taylor J.L., Jones J.D.G., Sandler S., Mueller G.M., Bedbrook J. Optimizing of expression of chimeric genes in plant cells // Mol. Gen. Genet. 1987. V. 210. P. 572-577.
Yamamoto Y. Y., Tsuji H., Obokata J. 5'-leader of a photosystem I gene in Nicotiana sylvestris, psaDb, contains a translational enhancer // J. Biol. Chem. 1995. V. 2705. P. 12466-12470.